EN ISO 13485 Certification

Quality Management System EN ISO 13485 certification
Implementing and maintaining a robust quality management system (QMS) for your medical devices, products and services provides an indispensable foundation on which to build your business. EN ISO 13485 certification of your QMS demonstrates your commitment to operating at a global standard.
The EN ISO 13485 certification process includes on-site audits to verify the capability and reliability of your quality management system. Our experts assess both the practical application and degree of effectiveness in the areas of design, development, production and customer care.
As a well-respected and globally recognized Notified Body, we hold extensive accreditations and are able to serve you at regional facilities worldwide. Our one-stop-shop portfolio consists of comprehensive services designed to accommodate your unique circumstances and business needs.
Optimize your QMS to the global standard of excellence!
Benefits of EN ISO 13485 certification
Medical devices (including Class I) greatly benefit from a production line, that includes an internationally recognized EN ISO 13485 certified quality management system (QMS). The certification framework provides for more product opportunities and extensive market access approval. Manufacturers and sub-contractors of medical devices can leverage their certified QMS status for a seamless transition into specialized certifications such as TCP, MDR, IVDR, and MDSAP. Start-up businesses benefit from the strong position EN ISO 13485 certification provides for future growth and expansion. Mindful preparation for the audit can also serve as an opportunity for all those in the medical devices industry to further refine QMS processes and workflows.
Four steps to EN ISO 13485 certification:
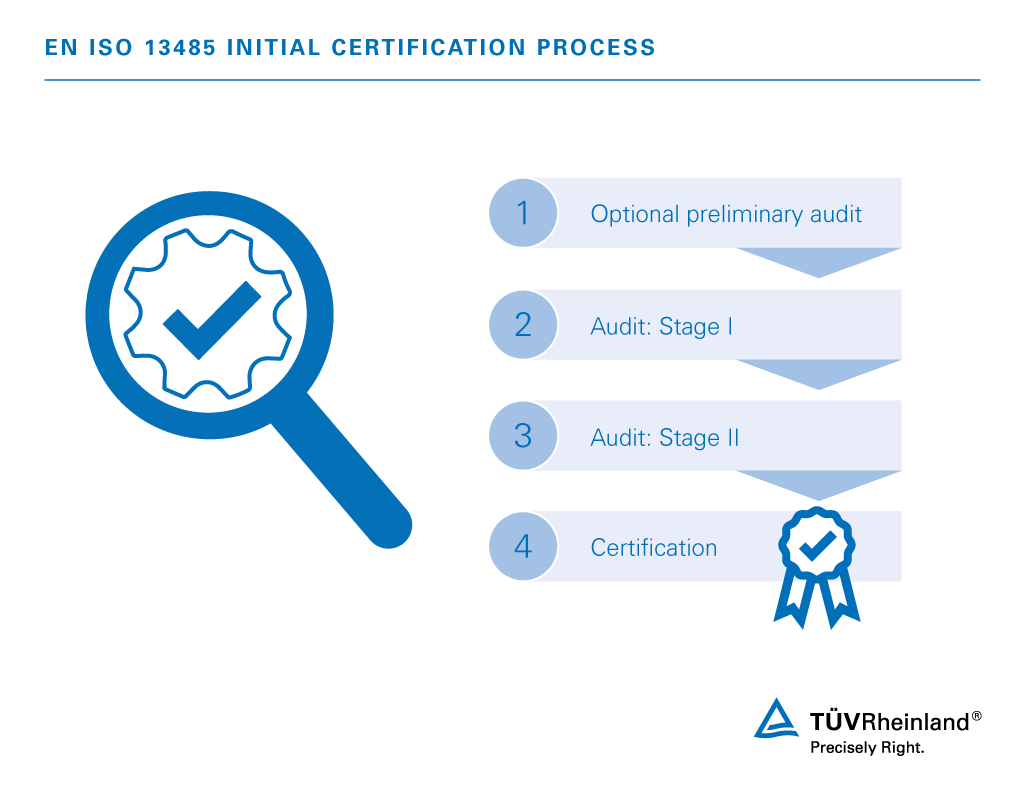
- Optional preliminary audit
Voluntary site inspection and quality management documentation review assessing your readiness for full-scale Phase I & II auditing. - Audit: Stage I
Assessment of certification eligibility determined by results of on-site audit, quality management documentation analysis and business assessment. - Audit: Stage II
On-site evaluation of quality management system for excellence in areas of applied practice and efficiency. - Certification
Official confirmation certifying the integrity of your quality management system and complete compliance to the standards.
First-time applicants benefit greatly from opting to undergo the preliminary audit. The process facilitates timely and effective preparation for the official EN ISO 13485 audit process. You are provided documented results that can be immediately applied for making adjustments in your processes prior to commencing with the mandatory audits.
Transfer of Certification applications are also being accepted, in addition to applications for Certification Renewal.
Our services are conducted using the four-eyes principle, which mandates the evaluations by the audit team and product experts be followed by an independent review for final decision.
Choose an experienced partner for your certification
We are a globally operational Notified Body with a wide range of accreditations and certified staff serving an extensive list of customers from every industry. Our EN ISO 13485 experts are here to serve you and your business around the world. Have confidence in your certification options and market access knowledge. Build your ideal certification package from a comprehensive list of services from a single provider.
SAC ISO 13485
Effective January 1, 2025, the Health Sciences Authority (HSA) in Singapore will only accept ISO 13485 certificates issued by Certification Bodies accredited by the Singapore Accreditation Council (SAC) or MDSAP certificates for medical device dealers seeking to renew or apply for their licenses. TÜV Rheinland Singapore Pte. Ltd. is an SAC-accredited Certification Body authorized to facilitate the certification of companies to the SAC ISO 13485 standard.
Contact


_core_4_3.jpg)
/tuv-rheinland-de19_p05_ivd09-lp_core_4_3.jpg)