IEC 60601-1 Medical Electrical Equipment
_core_2_2_1.jpg)
IEC 60601-1 Medical Electrical Equipment Testing and Certification
Mark your active medical devices as safe and reliable
Medical products must complete compliance evaluation, testing and device approval before being placed on the market.
Oversight of key compliance standards can be costly to the manufacturer, especially in the areas of product redesign, compliance testing, device approval and certification turnaround time.
Our experts offer clarification on IEC 60601 series for your desired markets, in addition to the framework in which your product design and manufacturing will be evaluated. IEC 60601 series are harmonized standards recognized worldwide with several localized deviations in key markets.
Expand your market access! Certify your active medical products.
Your IEC 60601-1 compliance for global market access
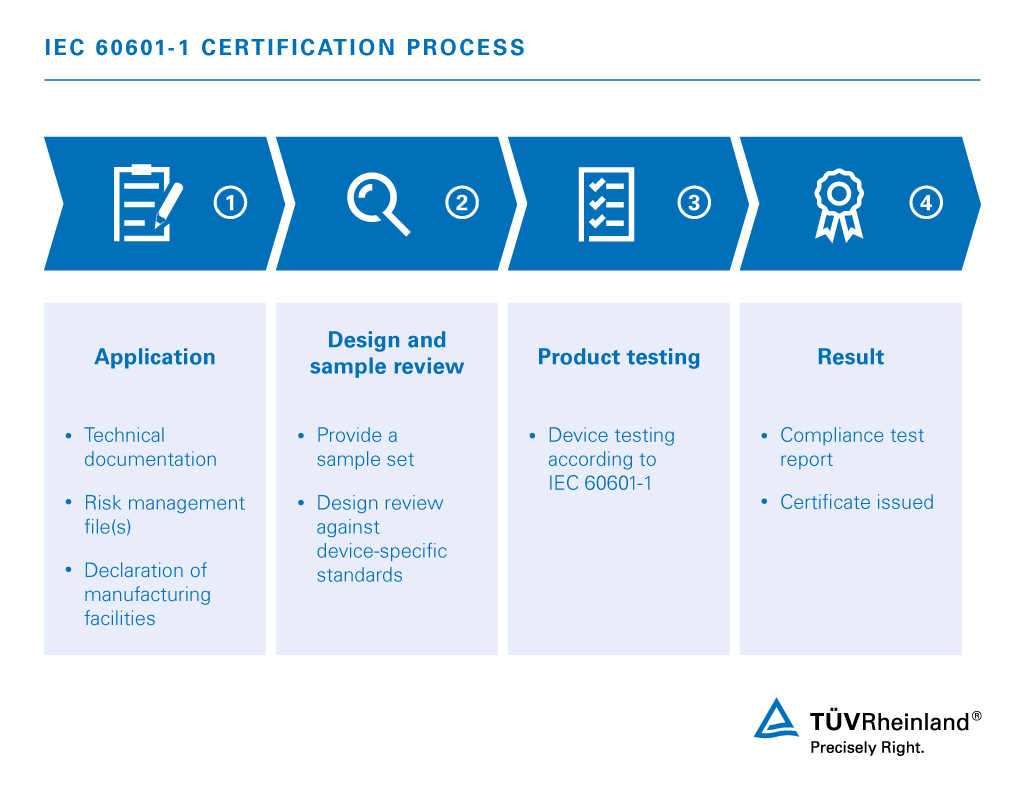
The testing and certification process of fulfilling the current IEC 60601-1 standard is a complex and multifaceted task. IEC 60601-1 mandates collateral, particular and performance standards specific to the device type, all of which are required for relevant certification schemes.
Documentation of ISO 14971 compliant risk management practices must be clear throughout the product lifecycle and device labeling. In conjunction with technical documentation and device sample, your medical electrical equipment and systems are tested according to established electromagnetic compatibility (EMC) as well as, electrical and environmental safety standards.
Your intended destination markets determine whether you will require a IEC 60601-1 certificate, a comprehensive evaluation report or both.
Our experts actively participate in standardization committees to gain unique technical expertise and actively stay informed of the latest changes to compliance requirements. Speak to an expert to determine if your device is eligible for testing or requires full IEC 60601-1 certification.
Choose an experience partner for your IEC 60601-1 compliance
Comprehensive compliance testing of your medical electrical equipment and systems ensures the safety, quality and performance of your products. Our experts can help you understand the specific IEC 60601-1 standard requirements relevant to your unique product.
Advantages with TÜV Rheinland:
- Upfront pricing
- Wide scope of comprehensive testing services
- State-of-the-art laboratories around the world for faster, reliable results
- Single source provider with quick reaction time from anywhere in the world
- Customized service packages tailored to your needs, saving you time and money.
TÜV Rheinland is one of the biggest medical scope providers worldwide. Our accredited laboratories are equipped with a wide-range of active medical device testing systems and are ready to serve you during your IEC 60601-1 certification. Choose a single source provider, receive all documents from one place, speak to experts around the clock, save time & money and get market-ready, fast.
Our market access services and multi-market certification programs, such as cTUVus Certification, CE Marking, INMETRO and CB scheme solutions for strategically entering and/or strengthening your position in competitive markets around the world.
Why TÜV Rheinland?
Our team of experts from Active Medical Devices, Medical Electrical Equipment IEC 60601-1 specialty, and Market Access Services are here to serve you and your business around the world. Have confidence in your certification options and market knowledge. Build your ideal certification package from a comprehensive list of services through a single-source provider.
We are a globally operational Certification Body with a wide range of accreditations, testing facilities and certified staff serving an extensive portfolio of customers from every industry.
Frequently asked questions
Regulatory testing standards can be complex and confusing. Here are just a few answers to some common questions. Our experts are happy to answer any further questions you may have. Speak to an expert!
Medical Electrical Testing Services
Take a look at our laboratory in Nuremberg, Germany. Watch the video now!
Additional Information
It is also important to see if any other applicable directive and standards are applicable. It is necessary to show compliance to all applicable standards and directives/norms before placing the product in the market.
In general most of the active electrical medical device needs to show compliance to IEC/EN 60601 series of standard. IEC/EN 60601-1 provides the General requirements for safety. Along with IEC / EN 60601-1 standard there are many collateral standards such as IEC/EN 60601-1-1: Safety requirements for medical electrical systems, IEC/EN 60601-1-2: Electromagnetic compatibility (EMC) - Requirements and tests, IEC/EN 60601-1-4: Programmable electrical medical systems, IEC/EN 60601-1-8: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems etc... Apart from the collateral standards there are particular part 2 standards based on the type of medical device and provides the specific requirement of medical device. For example IEC/EN 60601-2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment, IEC/EN 60601-2-19: Particular requirements for the safety of baby incubators etc.
The major concern in medical device compared to other products is leakage current, where the limits are more stringent than other products requirements. Also performance requirements form part of safety in medical device. Following are the various types leakage currents need to be evaluated in medical device:
- Earth leakage current
- Enclosure leakage current
- Patient leakage current
- Patient auxiliary leakage current
Based on the type of connection to patient, patient access points are defined as the applied parts. These are classified as
- Type B applied part
- Type BF applied part
- Type CF applied part
Parts of medical device which are intended to be connected to the patients during defibrillation procedure must qualify as defibrillation proof applied parts. Type BF / CF Applied parts which have protection against defibrillation needs to be evaluated additionally for defibrillation protection. This can be done with the help of the defibrillation simulator according to the IEC/ EN 60601-1, IEC/EN 60601-2-27, IEC/EN 60601-2-25, IEC/EN 60601-2-51, IEC/EN 60601-2-49 and AAMI standards such as EC 13, EC 11, EC12 etc..,. Basically a high voltage which is similar to defibrillation voltage ( up to 5KV) is applied across the leads which are intended to be connected to the patient and at the same the residual voltage on the enclosure other signal ports because of defibrillation is measured. The residual voltage should not be high enough so that the operator and other personnel near patient are safe in terms of electric shock.
TUV Rheinland India has in-house capability to test and evaluate medical device which are having defibrillation applied parts according to most of IEC/EN 60601 and AAMI standards
Test Specification
The defibrillation test equipment Defib-5 can be configured to provide the circuits as per IEC/ EN 60601-1, IEC/EN 60601-2-27, IEC/EN 60601-2-25, IEC/EN 60601-2-49 and AAMI standard EC 13 with the following specification
- Voltage: ± 5000Vdc
- Voltage wave form: 20x2200µsec
Light based therapeutic or diagnostic medical device such as infant radiant warmers and infant phototherapy equipment shall be evaluated for Ultra violet (UV) and Infrared (IR) irradiation according to respective standards IEC/EN 60601-2-21 and IEC/EN 60601-2-50. During this test it is evaluated that the infant patient is not getting exposed to the dangerous UV and IR radiations.
TUV Rheinland India has specified measuring equipment, which can measure UV/IR irradiation in the range of 180nm to 2100nm
Test Specification
The test equipment ILT 1700 is a light measuring device with the different type of detectors. The detectors SED 623 is an IR detector and SED 240 is UV detector in the following wavelength range
- Measuring wavelength range of SED623: 438-2100 nm
- Measuring Wavelength range of SED240: 180-400 nm
If the product is targeted for global market where different countries required different approvals then it is recommended to have CB report and certificate which helps to get various country approvals. IS 13450-1 is equivalent Indian standard for IEC 60601-1.
Similarly electrical In Vitro Diagnostic Device needs to be tested according to IEC/EN 61010-1 along with particular standard IEC/EN 61010-1-101. Electro Magnetic Compatibility (EMC) needs to show compliance mainly according to IEC/EN 61326.
Latest News
TÜV Rheinland’s services for ECG machines
TÜV Rheinland (India) Pvt. Ltd. offers manufacturers /Design houses to test their single or multichannel ECG as per IEC/EN/AAMI standards. TÜV Rheinland India has complete in-house test facilities for diagnostic, ambulatory or monitoring ECG system as per IEC 60601-1, IEC 60601-2-25, IEC 60601-2-47 and IEC 60601-2-27 and AAMI/ANSI EC11, EC13 standards.
Our CBTL and NABL accredited laboratories have capabilities to perform below tests for ECG machines for their international market access:
Recovery time, overload tolerance, channel crosstalk, distortion, tests with sinusoidal and impulse signals, high frequency response, low frequency response, linearity and dynamic range, record identification, patient identification sensitivity, frequency response, input impedance, pacemaker rejection, defibrillation protection for common and Differential-mode test, etc.,
Implementation of IEC/EN 60601-1 3rd edition
- Represents new state of the art for safety requirements
- Compliance can be presumed to verify acceptable risk unless there’s objective evidence to the contrary
- General requirement for design process to include a RISK MANAGEMENT process in accordance with ISO 14971
- Safety has been broaden from BASIC SAFETY to include ESSENTIAL PERFORMANCE
- 3rd Edition <--> 2nd Edition Mapping Document
- IEC TR 62348
- IEC 60601-1: General Requirements for Safety, Part 1
- Collaterals Inserted into General Standard, 3rd Edition
- Safety Philosophy, Clause 4.1, 4.2, 4.7
- “…Requirements shall apply in NORMAL USE and reasonably foreseeable misuse”
- “A RISK MANAGEMENT PROCESS complying with ISO 14971 shall be performed”
- Residual RISK must be acceptable
- “ME Equipment shall … be SINGLE FAULT SAFE” (free of unacceptable RISK under SINGLE FAULT CONDITION)
- Safety Philosophy, 4.3
- “Manufacturer identifies which functions … are Essential Performance”
- Determined by manufacturer’s policy for Risk acceptability
- “Collateral and Particular Standards [aligned with 3rd edition] are expected to identify specific Essential Performance,” Annex A, 3.27
- Safety Philosophy, Clause 12.2
- Address in a USABILITY ENGINEERING PROCESS the RISK of poor USABILITY
- Equivalent Safety
- “Equivalent Safety” clause
- 2nd edition, Clause 3.4; 3rd edition, Clause 4.5
- Alternative means of addressing risks are acceptable provided that residual risk from applying the alternative means and the verifiable requirements in the standard are equivalent or the alternative means is better (less risk).
Contact


_core_4_3.jpg)
_core_4_3.jpg)

