Our Expertise for Your Successful Market Entry in Europe
In vitro diagnostic medical devices (IVD) are used to analyze human body samples for medical purposes. The term covers products as diverse as reagents, calibration materials, control materials, kits, instruments, systems and software. The results of tests where IVDs are used influence medical decisions directly affecting patient wellbeing therefore, the safety and reliability of these medical devices is of particular importance.
Since 1998, in vitro diagnostic medical devices have been regulated by Directive 98/79/EC. On May 25, 2017, the new EU Regulation on In Vitro Diagnostic Medical Devices (IVDR) 2017/746 came into force, placing more extensive requirements on IVD and their manufacturers. Following a five-year transition period, products must meet the requirements of the IVDR in order to receive the CE mark and be allowed to enter the European market. (A longer transition period applies to a few exceptions.) It is noteworthy that CE marking provides a competitive advantage in many markets worldwide.
As one of currently twelve Notified Bodies worldwide (status: July 30, 2024), we offer services throughout your transition to the new IVDR. As a single source provider with a comprehensive range of medical testing and certification services, we can offer you a customized service tailored to your specific products and needs.
You want to learn more about our services related to the IVDR?
Key Update on EU Regulation 2024/1860: Extended Transition Periods and New Requirements for IVDs
On July 9, Regulation (EU) 2024/1860 was published in the Official Journal of the European Union and entered into force. The regulation amends regulations (EU) 2017/745 (MDR) and (EU) 2017/746 (IVDR) and covers the following three topics:
• The gradual roll-out of Eudamed as the different modules become available
• The obligation of manufacturers to inform the public in case of interruption or discontinuation of supply
• Transitional provisions for certain in vitro diagnostic medical devices (IVDs).
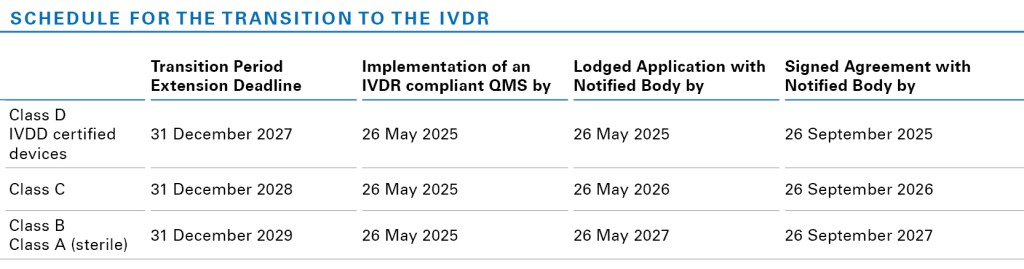
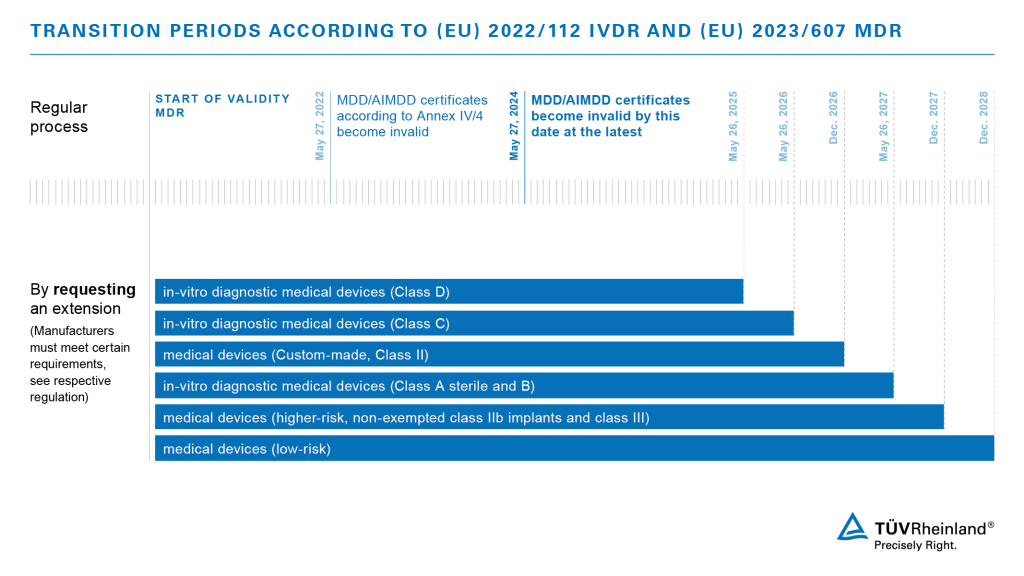
One of the most significant changes resulting from the new regulation is the extension of the IVDR transition period for legacy devices already supplied under the Directive 98/79/EC (IVDD). Devices that have been withdrawn from the scope of IVDD certification or that have undergone a significant change cannot benefit from the new transitional provisions unless specific conditions are met.
The new transitional provisions ensure continuity of supply of IVDs to the market, while giving manufacturers and Notified Bodies time to approve legacy devices under the IVDR. Manufacturers of legacy IVDs must have a signed agreement with their Notified Body for a conformity assessment and have lodged an application for IVDR certification to benefit from the extended transition periods. They must also have implemented QMS requirements under the IVDR before May 26, 2025.
As one of the first Notified Bodies to receive notifications for IVDs under the IVDR, TÜV Rheinland welcomes the new timeframes and will support customers during the extended transition process. It is important that manufacturers continue to work towards the transition to avoid Notified Bodies being overloaded with review and certification under the new timeframes. TÜV Rheinland will prepare all relevant procedures and templates to ensure a smooth transition and keep you updated.
Transition Period for the EU Regulation IVDR 2017/746 | TÜV Rheinland
All IVDR Services
With 40 years of experience as a provider of testing services for the safety and quality of in vitro diagnostic medical devices (IVD), we are a competent partner for transitioning to the IVDR. In addition, we support manufacturers of IVD and medical devices with market access services into the European and international markets.
Do you have any questions about the IVDR?
With our experience as Notified Body with the certification of in vitro diagnostic medical devices, we support you during the transition from the IVD Directive to the IVDR with meeting deadlines and with many other aspects that are relevant for your products to enter the European market.
Here, we have summarized the most important questions and answers for you.
Further Information
New regulation for in vitro diagnostic medical devices:




/tuv-rheinland-de19_p05_ivd09-lp_core_4_3.jpg)

/tuv-rheinland-in-vitro-diagnostic-devices-(ivd)-sk-297595064_core_4_3.jpg)